Patients' health
of DOZ Dbam o zdrowie
pharmacies have facilities
for the disabled
of Natura beauty stores
are accessible by people
with disabilities
private labels
at the Natura chain
private label products at Natura
by 8m
people
spent by the
DOZ Foundation
on its mission
new DOZ Products
Patients' health and safety
Pelion’s business philosophy is reflected in its new mission: ‘We are here to ensure a long and quality life for our customers and patients’.
All its activities are oriented towards the good of end customers, whether businesses or individuals. At each stage, the staff take care to provide top quality service. Every product goes a long way before reaching the hands of a patient or customer, and all employees, in every position, know and understand the rules and procedures adopted by Pelion. Observance of these rules is a guarantee that all products on the pharmacy and store shelves are safe and effective.
With the highest quality of service in mind, the Company established procedures regulating the supply chain processes. It also built modern infrastructure to ensure proper conditions of receiving, storing and dispensing pharmaceuticals. In their work, all employees are guided by ethical values and standards of conduct. Their work with others relies on trust, transparency, honesty and responsibility. It is of crucial importance that any threats, whether internal or external, are promptly responded to. Safety is the responsibility of wholesale managers as well as the complaint and control departments, which work closely with other divisions of the Company and with suppliers. The Quality Assurance Department, headed by the Quality Director, makes sure that all business processes are conducted properly, up to the required standards of quality.
Pelion Group companies are active in wholesale and retail trade in medicinal products, medical devices, foodstuffs for particular nutritional uses, dietary supplements, cosmetics, hygiene products, sick and baby care products, foodstuffs containing natural ingredients of plant origin listed in pharmacopoeias, and medical disinfectants admitted for marketing by pharmaceutical wholesalers, as specified in Art. 72.5 of the Pharmaceutical Law. The wholesale and retail trade is carried out through three business lines: wholesale, sales to hospitals and logistics services, and retail sales.
Pelion Group companies are involved in the product* life cycle at the receipt, storage, distribution and supply stages.
Key distribution processes are presented in the chart below:
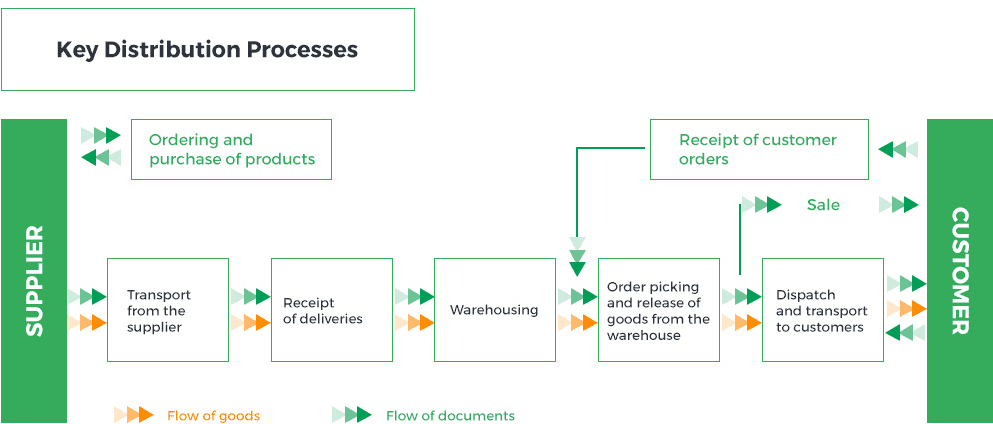
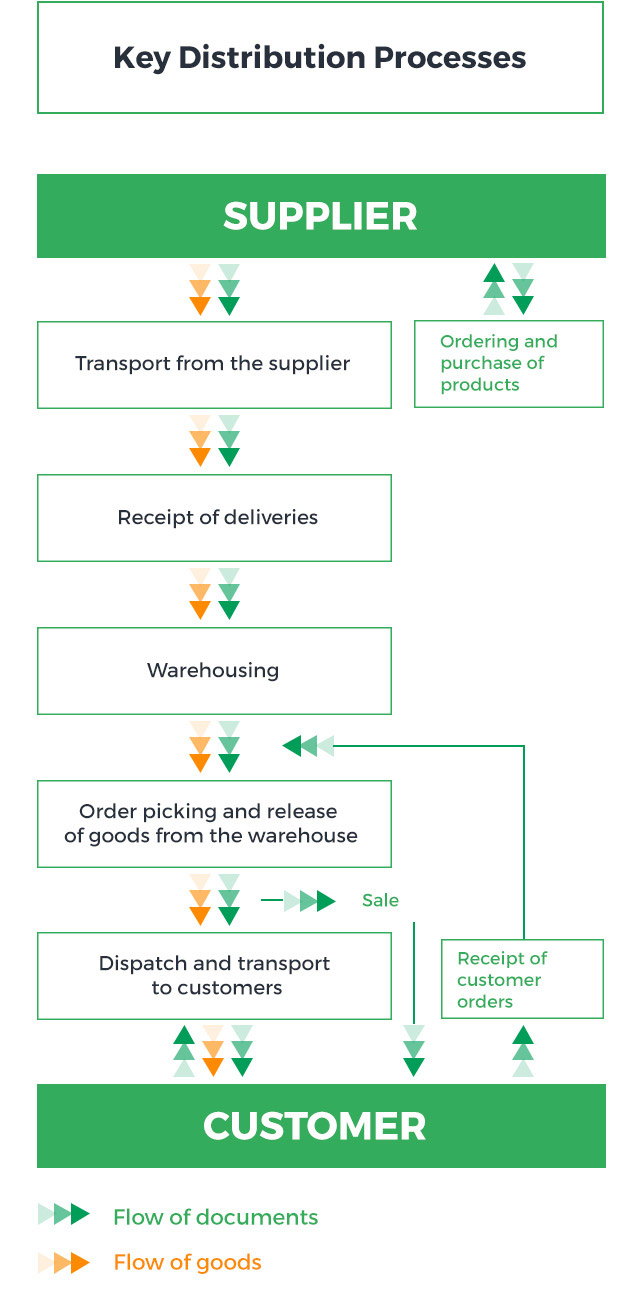
* Product is understood as the pharmaceuticals distribution service provided by Pelion Group companies.
All distribution processes are subject to the procedures of the Quality Assurance System, based on the requirements of Good Distribution Practice, and must comply with applicable laws, internal procedures and regulations.
At every stage of the distribution process, products of each group are handled in accordance with the following primary regulations:
- Pharmaceutical Law of September 6th 2001, as amended (Dz.U. of 2008, No. 45, item 271 − consolidated text),
- latest amendment: Act of September 25th 2015 amending the Pharmaceutical Law − Dz.U. of 2015, item 1771
- Act on Food and Nutrition Safety of August 25th 2006, as amended (Dz.U. of 2010, No. 136, item 914 − consolidated text),
- latest amendment: Act of November 28th 2014 amending the Act on Food and Nutrition Safety − Dz.U. of 2015, item 35
- Medical Devices Act of May 20th 2010, as amended (Dz.U. of 2015, item 876 − consolidated text),
- latest amendment: Act of September 11th 2015 amending the Medical Devices Act − Dz.U. of 2015, item 1918
- Cosmetics Act of March 30th 2001, as amended (Dz.U. of 2013, item 475 − consolidated text)
- Drug Addiction Prevention Act of July 29th 2005, as amended (Dz.U. of 2012, item 124 − consolidated text),
- latest amendment: Act of April 24th 2015 amending the Drug Addiction Prevention Act − Dz.U. of 2015, item 875
- Act on Reimbursement of Drugs, Foodstuffs Intended for Particular Nutritional Uses and Medical Devices of May 12th 2011 (Dz.U. of 2015, item 345 − consolidated text)
- Biocidal Products Act of October 9th 2015 (Dz.U. of 2015, item 1926).
Safe purchasing and storage
With a view to ensuring that products are delivered to customers in a safe manner and that the quality required for their designated use is maintained, Pelion:
- purchases products only after the required licences and authorisations have been verified and confirmed, and after the products have been entered in the Central Catalogue of Products,
- qualifies suppliers and customers upon confirmation of the required licences and authorisations based on the Central Catalogue of Suppliers,
- thoroughly checks compliance of delivered products with the purchase documentation (the scope of information checked depends on the type of products, i.e. for medicinal products it includes details of the supplier, name and country of the manufacturer, product name, batch number and expiry date, and, where an official fixed price or wholesale margin apply to the product, the price), and the transport conditions,
- monitors storage conditions,
- monitors transport conditions.
Pelion has state-of-the-art and well-engineered warehouses, where the processes and equipment are constantly improved to ensure high quality and speed of deliveries. The way the warehouses operate is monitored by way of internal audits and external inspections, e.g. by the Sanitary and Epidemiological Stations (SANEPID) and Sanitary Inspectorates.
Inspections carried out in 2015 revealed six instances of non-compliance at two warehouses, and were followed up by warnings.
Safe transport
Transport services (including transport of medicinal products) are provided in compliance with the following primary regulations:
- Road Transport Act of September 6th 2001, as amended (Dz.U. of 2013, item 1414 − consolidated text)
- Drivers’ Work Time Act of April 16th 2004, as amended (Dz.U. of 2012, item 1155 − consolidated text)
- Road Traffic Law of June 20th 1997, as amended (Dz.U. of 2012, item 1137 − consolidated text)
- Public Roads Act of March 21st 1985, as amended (Dz.U. of 2015, item 460 − consolidated text)
- Transport Law of November 15th 1984, as amended (Dz.U. of 2015, item 915 − consolidated text).
Road transport safety is ensured by:
- using qualified vehicles only,
- inspecting vehicles in accordance with the applicable procedure,
- loading products in accordance with the applicable procedure for release and transport of goods to customers,
- travelling controlled routes and monitoring transport conditions,
- unloading at safe locations (permitted locations only),
- unloading in accordance with the applicable procedure,
- following established procedures in the event of malfunctions or deviations,
- taking corrective and preventive actions.
Adjusting the facilities to customers’ needs
In its day-to-day operations, Pelion takes care of the needs of the most vulnerable. To ensure that its pharmacies and beauty stores are safe and accessible, the Company adjusts them to meet the needs of elderly customers, the disabled and people with small children. 547 DOZ Apteki dbam o zdrowie pharmacies and 226 Natura stores have access ramps and easy-access entrances, and where access is hindered, customers can call and ask for assistance.
New forms of communication
New forms of communication with patients are developed and constantly improved. Patients can use Pelion web portals and sites to look up information about products and their use, as well as to exchange information with experts in various fields of pharmacy and medicine. They can also write blogs and share experience, e.g. on medme.pl. Social media are a source of inspiration for patients, including the elderly. Profiles have been created, for example, for the cafesenior, Magazyn 60+, DOZ pl, DOZ Maraton Łódź in partnership with PZU, DOZ Foundation, Natura beauty stores, and Urtica for Children.


Customer satisfaction
One of the tools used by the Company to raise the quality of its services is the customer satisfaction survey. In 2015, the survey was carried out by an external company at Natura stores, using the mystery shopping method. The mystery shoppers wrote down their observations and comments on evaluation sheets, which contained a list of closed-ended questions. The survey was conducted at all proprietary and agency stores across Poland, every month from February to November.
The objective of the survey was to evaluate the facilities in the following six aspects:
- Interior and surroundings (first impression, product display and presentation)
- First impression (greeting, first contact)
- Sale (asking open-ended questions, offering, presenting products, cross-selling)
- At the check-out counter (encouragement to buy product of the day, payment, goodbye)
- General impression (interest in customer, friendliness, politeness)
- POS materials (presence of marketing materials).
The survey was to help the facilities achieve the result of at least 90% over the 10 months.
Month by month, the results were as follows: February 2015 – 83.4%, March 2015 − 88.6%, April 2015 − 88.2%, May 2015 − 88.9%, June 2015 − 88.3%, July 2015 − 88.1%, August 2015 − 88.6%, September 2015 − 93.5%, October 2015 − 92.2%, November 2015 − 87.39%. The 10-month average was 88.7%.
After an in-depth analysis of individual aspects, measures were taken to improve the results. Managers responsible for the stores held one-to-one or group workshops to help them better tailor their offerings and services to customer needs and expectations.
In 2015, DOZ S.A. also decided to carry out a survey of customer satisfaction to assess, among other things, how orders were processed at DOZ.pl. After each completed order, the customer was asked to fill in a questionnaire about their satisfaction with using the portal. The survey is in progress.
Proprietary brands
The retail companies offer private label products. The DOZ Product label, launched in 2015, is a response to Patients’ expectations of high quality at affordable prices. DOZ Product is a label covering three brands (ca. 50 products in 2015), all developed in consultation with doctors and pharmacists. Natura has 12 proprietary brands (1,412 products). To take greater care of customers’ health, the company began to verify the composition of products sold at its beauty stores. It wants to offer cosmetics with mostly natural ingredients (e.g. the Stara Mydlarnia, Sylveco, Green Pharmacy brands) and as little preservatives as possible. To ensure that all products offered to customers are safe, the company established the following procedures:



These measures guarantee that products of unsatisfactory quality are removed from the shelves and customer safety is maintained. Foodstuffs, OTC drugs and medical devices sold at Natura beauty stores are subject to special supervision. These products are delivered directly to individual stores, to minimise the risk of quality deterioration due to inadequate conditions in transport. All products are examined upon delivery, and if found unsatisfactory, they are not accepted into stock. The HACCP system is a tool used to meet the safety and health quality requirements for foodstuffs, and the constantly developed Good Distribution Practice is applied to drugs and medical devices. With children’s safety in mind, drugs are displayed at heights where children cannot reach them. Furthermore, products are divided into groups, so that no unwanted smells (e.g. of detergents) get to food.
Customer privacy
Nowadays, protection of information is a foundation of trust and loyalty, as well as a proof of service quality. Data protection at the Company is regulated by the Information Security Policy, which ensures that information flow is properly secured, any irregularities are monitored, and no unauthorised access to data is allowed. No breach of customer privacy or data loss at the Company was reported in 2015.

Created by John Pitcher